The following is reproduced from an editorial article by Kamal Pratap Singh published in Biotech Express on page 8 in November 2024 based on the webinar ‘The Global Burden of Rare Diseases: Issues and Challenges’ addressed by Prof. Ramaiah Muthyala at the Federation of Asian Biotech Associations (FABA)-US Chapter.
In a ground-breaking webinar hosted by the Federation of Asian Biotech Associations (FABA)-US Chapter, Prof. Ramaiah Muthyala, President and Founder of the Indian Organization for Rare Diseases (IORD), and Research Associate Professor at the University of Minnesota, USA, delivered an inspiring address titled “The Global Burden of Rare Diseases: Issues and Challenges.”
The session highlighted the critical, often overlooked, public health crisis posed by rare diseases (RDs) in India, while also exploring lessons from global practices, particularly in the United States.
Although rare diseases are individually uncommon, collectively they affect a staggering 70 million people in India and over 350 million worldwide. These conditions, often referred to as orphan diseases, present immense socio-economic and emotional challenges for patients and their families. The discussion underscored the urgent need for innovative policies, equitable healthcare frameworks, and collaborative efforts to address this escalating crisis.
Rare Diseases: A Global and National Perspective
Prof. Muthyala began by placing India’s rare disease challenges within a global context, emphasizing disparities in healthcare access, costs, and policy implementation. In the United States, rare diseases have long been a focus of public health policy, with the Orphan Drug Act of 1983 catalyzing significant advancements in research, drug development, and patient advocacy. This act incentivized pharmaceutical companies through tax credits, grants, and extended market exclusivity, leading to the approval of over 1,000 orphan drugs.
Despite these advancements, the exorbitant costs of orphan drugs remain a critical issue globally. For example, therapies like Zolgensma (for spinal muscular atrophy) can cost upwards of $2.1 million per patient, while daily medications for ultra-rare conditions can exceed $250,000 annually. Insurance coverage, though more accessible in high-income countries, often falls short, leaving many families financially devastated.
In contrast, India faces even more significant challenges. While the country is a global hub for generic pharmaceutical production, it heavily relies on imports for rare disease drugs, which are often unaffordable. For instance, Trientine, a therapy for Wilson’s disease, costs ₹1.6 crore per year when imported, making it out of reach for most Indian families, whose average monthly income is under $300.
India’s Rare Disease Landscape
India’s rare disease ecosystem remains in its early stages, with limited diagnostic infrastructure, underdeveloped registries, and fragmented policy implementation. Prof. Muthyala noted that only 14,994 cases have been recorded in the ICMR National Registry for Rare and Other Inherited Disorders—just a fraction of the estimated burden. Diagnostic delays, averaging 7 to 20 years, further exacerbate patient suffering.
While government initiatives like the National Policy for Rare Diseases (2021) have been introduced, progress has been slow. Prof. Muthyala pointed out that only 48.7% of allocated resources for Centers of Excellence (CoEs) were utilized between 2021 and 2024. Additionally, bureaucratic inefficiencies and a lack of centralized coordination have hindered the policy’s impact.
However, there have been strides. India now manufactures 450 active pharmaceutical ingredients (APIs) for rare disease therapies, thanks to initiatives like Make in India and the ₹15,000 crore Production Linked Incentive (PLI) scheme. This has the potential to reduce costs and improve accessibility, though systemic barriers remain.
The Role of IORD: Advocacy, Innovation, and Collaboration
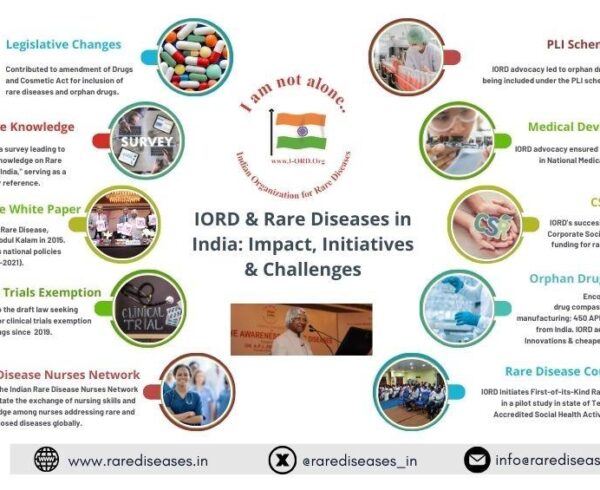
Under Prof. Muthyala’s visionary leadership, the Indian Organization for Rare Diseases (IORD) has been at the forefront of transforming India’s rare disease landscape. Founded in 2005, IORD has spearheaded several groundbreaking initiatives. The organization has played a pivotal role in policy advocacy, contributing significantly to the formulation of the National Policy for Rare Diseases and championing the inclusion of orphan drugs under initiatives like the Production Linked Incentive (PLI) scheme and the National Medical Devices Policy (2023).
IORD’s commitment to raising awareness is exemplified by the landmark White Paper on Rare Diseases, introduced in 2015 by Dr. APJ Abdul Kalam, which continues to serve as a cornerstone for policy development. Furthermore, IORD has fostered global collaborations, enabling India to engage in advanced research areas such as genome editing and genetic therapies, bridging gaps in innovation and treatment accessibility.
Additionally, IORD has launched innovative projects like the Rare Disease Counting Pilot Project in Telangana to improve data collection, and the Indian Rare Disease Nurses Network, which provides specialized training to healthcare professionals.
Lessons from the USA and Global Collaborations
The webinar also explored global best practices, emphasizing the success of the U.S. in fostering an ecosystem for rare disease research and treatment. Prof. Muthyala highlighted the role of the Office of Orphan Products Development (OOPD) at the FDA, which streamlines research, clinical trials, and regulatory approvals for orphan drugs. Establishing a similar office in India could accelerate progress and ensure treatments are both accessible and affordable.
Global collaborations remain a key pathway forward. Prof. Muthyala stressed the importance of public-private partnerships, international funding mechanisms, and community-driven initiatives to build an inclusive and sustainable rare disease ecosystem.
Call to Action: Building an Inclusive Future for Rare Diseases
Prof. Muthyala concluded with a powerful call to action. He urged policymakers to adopt a multi-stakeholder approach, bringing together governments, healthcare providers, patient advocacy groups, and pharmaceutical companies to address rare disease challenges holistically.
“Rare diseases may affect a small percentage of individuals, but their impact on families and communities is profound. We must think like health economists, not just scientists, to develop sustainable solutions that leave no patient behind,” he stated.
This webinar has set the stage for actionable insights and renewed hope for millions of rare disease patients in India. With sustained focus, innovation, and collaboration, the vision of equitable healthcare for all, championed by IORD, can become a reality.
Experts Call for Unified Efforts to Transform Rare Disease Care in India
The webinar brought together leading scientists, industry pioneers, and healthcare professionals to address the pressing challenges of rare diseases in India. The discussions shed light on the critical gaps in treatment, research, and patient care, while outlining a collective vision for the future.
Dr. Mohammed Ansari, Director of Business Development at ClinRé, raised a crucial point about the challenges of developing therapies in a country where patient numbers are limited and few are registered. He emphasized the difficulty in assessing the efficacy of orphan drugs under these constraints, calling for innovative solutions tailored to India’s unique healthcare landscape.
Highlighting the need for systemic reform, Prof. Muthyala underscored the importance of collaboration between the government, policymakers, pharmaceutical companies, and patient advocacy groups. He stressed that building robust patient registries is essential to improving treatment access. Additionally, he advocated for policy initiatives to reduce costs and urged Indian manufacturers to unite in producing affordable orphan drugs to benefit underserved populations.
The event also attracted international interest, with Mr. Denis Demarais, Managing Director of Rius Medical UG, expressing his readiness to collaborate with Indian companies. He proposed partnerships to manufacture and distribute affordable orphan drugs, which could pave the way for transformative healthcare accessibility in India.
Dr. Jugnu Jain, CEO of Sapien Biosciences, Hyderabad, pointed to a significant gap in India’s rare disease landscape: the absence of biobanking facilities for patient samples. While cancer care in India benefits from robust registries, she noted that rare disease research lags due to poor awareness and inadequate infrastructure. She highlighted the value of biobanks as a vital resource for advancing clinical studies and improving patient outcomes.
Sharing insights from his work on craniofacial deformities, Dr. Eswarakumar Jacob, Founder and CEO of Krouzon Pharmaceuticals, USA, detailed the staggering costs of rare disease management, often exceeding $1 million per patient. He revealed his company’s efforts to mitigate these expenses by donating 10% of its profits to pediatric patients and registering a subsidiary in India to partner with NGOs for free drug distribution.
The session concluded with Dr. Vadlamudi Raghavendra Rao, Dean of Research at the Genome Foundation of India, emphasizing the critical role of early screening and diagnosis in reducing the burden of rare diseases. Drawing on his expertise in genomics and genetic diseases, he stressed the need for comprehensive patient registries at national, regional, and local levels. These registries, he argued, could drive policy changes, enhance awareness, and promote ethical practices in disease prevention and management.
Check the link for full story