The following is the transcript of the address delivered by Prof. (Dr.) Ramaiah Muthyala, President & CEO, Indian Organization for Rare Diseases (IORD), Professor & Director, University of Minnesota, USA on the theme “Healthy Beginnings, Hopeful Futures: Rare Diseases and the Global Call to Action”, presented during the 342nd International Webinar organized by RJS PBHRead more
Blog
Vijayawada: The Indian Organization for Rare Diseases (IORD), a non-profit advocacy body, convened a critical conference titled “RAISE THE AWARENESS – RARE DISEASES: Advocate Public Policy, Promote Diagnosis, Treatment & Social Services” at Fortune Murali Park, Vijayawada on February 28, to mark World Rare Disease Day 2025. The event, attended by healthcare experts, policymakers,Read more
This is a transcribed speech of Indian Organisation for Rare Diseases (IORD) President and CEO, Prof. Ramaiah Muthyala delivered at the World Rare Disease Day 2025 conference in Vijayawada, organized by the IORD. Check the full video here. Today, while India celebrates National Science Day, the world celebrates Rare Diseases Day. Both are important,Read more
This is a transcribed speech of Sri M.T. Krishna Babu, IAS, Special Chief Secretary, Health Medical & Family Welfare, Govt. of Andhra Pradesh highlighting need for Strengthening Rare Disease Care in Andhra Pradesh. He delivered this speech at the World Rare Disease Day 2025 conference, organized by the Indian Organisation for Rare Diseases in Vijayawada,Read more
Manufacturing Drugs for Selected Rare Diseases: Dr Vinod K Paul This is a transcribed speech of Dr. Vinod K. Paul, Member of the National Institution for Transforming India (NITI) Aayog, on “Manufacturing Drugs for Selected Rare Diseases.” He delivered this speech at the World Rare Disease Day 2025 conference, organized by the Indian Organisation forRead more
Registration is now open for World Rare Disease Day-2025 Conference, organized by the Indian Organisation for Rare Diseases (IORD) at FORTUNE Murali Park, Vijayawada in Andhra Pradesh on 28 February, 2025. This significant event aims to advance healthcare innovation, address rare diseases, and strengthen collaborative efforts in public health. To register for the conference, pleaseRead more
New Delhi: In the run up to the World Rare Disease Day 2025 on 28 February, a 20-day awareness campaign for rare diseases was launched by the Ram-Janki Institute Positive Broadcasting House (RJS PBH), led by Founder and National Coordinator Uday Kumar Manna. The event – starting with a webinar on “Urgent need for aRead more
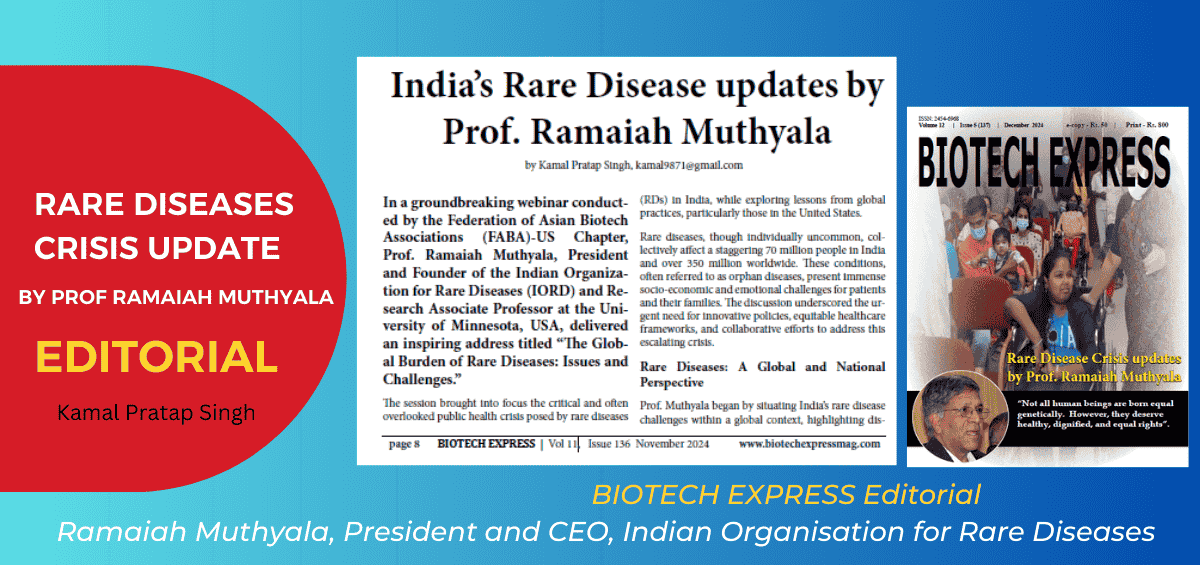
The following is reproduced from an editorial article by Kamal Pratap Singh published in Biotech Express on page 8 in November 2024 based on the webinar ‘The Global Burden of Rare Diseases: Issues and Challenges’ addressed by Prof. Ramaiah Muthyala at the Federation of Asian Biotech Associations (FABA)-US Chapter. In a ground-breaking webinar hostedRead more
In a landmark ruling, the Delhi High Court ordered the creation of a ₹974 crore National Fund for Rare Diseases for 2024–26, emphasizing patient-centric policies, expanded treatment access, and funding reforms.
The Delhi High Court has introduced a landmark Standard Protocol to streamline rare disease management, ensuring continuous availability of therapies, local drug manufacturing, and time-bound treatment delivery.