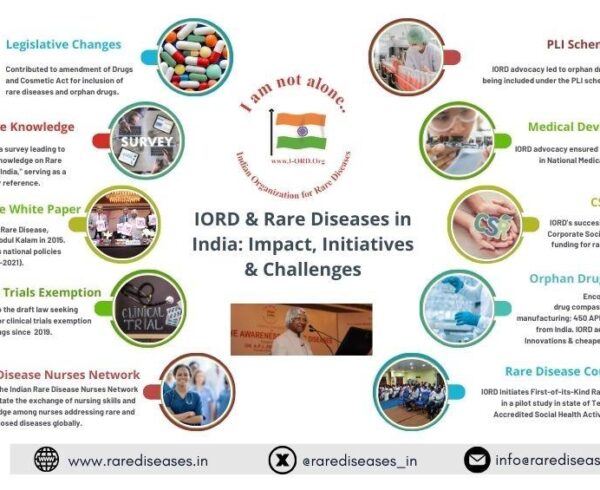
When the Union Cabinet, chaired by Prime Minister Shri Narendra Modi, approved the National Medical Devices Policy, 2023 on April 26, it came as a shot in the arm for rare disease advocacy Indian Organisation for Rare Diseases (IORD).
This policy aims to promote the growth of the medical devices sector in India and achieve a market size of $50 billion in the next five years, up from the current $11 billion. The policy gives special importance to rare diseases, which are clearly mentioned in the new approved medical devices policy in section 41 (4) of the New Drugs, Medical Devices, and Cosmetics Bill. It states that the Central Licensing Authority may, in the public interest, abbreviate, defer, or waive pre-clinical and clinical data requirements for the approval of new drugs related to life-threatening or serious diseases, rare diseases, or diseases of special relevance to the country.
In this regard, IORD took steps and brought it to the attention of key stakeholders by writing to NITI Ayog member Dr. Vinod Kumar Paul, the Federation of Indian Chambers of Commerce and Industry, and the Association of India Medical Device Industry. In emails and letters dated November 18, 2021, IORD CEO & President Prof. Ramaiah Muthyala highlighted how orphan drugs have gained significant attention in recent years, but medical devices for rare diseases have not received equal recognition, despite there being 90 million rare disease patients in India and 300 million worldwide.
The medical devices sector has played a crucial role in combating the COVID-19 pandemic by producing essential medical devices and diagnostic kits. The policy focuses on several strategies to support the sector’s growth, including streamlining regulations, creating enabling infrastructure, promoting research and innovation, attracting investments, and developing skilled manpower. It also emphasizes brand positioning and awareness creation through the establishment of an Export Promotion Council and learning from global best practices.
By implementing this policy, the government aims to make the medical devices industry self-reliant, resilient, and competitive, catering not only to India’s healthcare needs but also to the global market. The Government of India has already initiated the implementation of the PLI Scheme for medical devices and support for setting up 4 Medical Device Parks in the states of Himachal Pradesh, Madhya Pradesh, Tamil Nadu, and Uttar Pradesh. Read more details here.